Co2 Lewis Dot
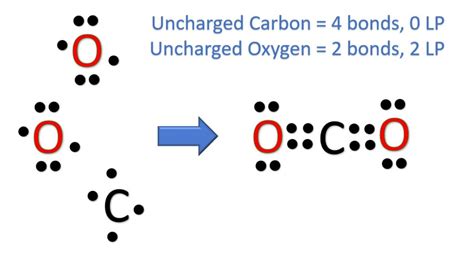
The CO2 molecule, also known as carbon dioxide, is a fundamental compound in chemistry, playing a crucial role in various biological and industrial processes. To understand its structure and properties, it's essential to examine its Lewis dot representation. The Lewis dot structure, developed by Gilbert N. Lewis, is a convenient way to depict the valence electrons in molecules, providing insights into their bonding and reactivity.
Understanding the CO2 Lewis Dot Structure
To construct the CO2 Lewis dot structure, we start by determining the total number of valence electrons in the molecule. Carbon © has 4 valence electrons, and each oxygen (O) has 6. Therefore, the total number of valence electrons in CO2 is 4 (from C) + 6*2 (from two O atoms) = 16 electrons. The next step involves drawing the skeletal structure of the molecule, where carbon is typically the central atom due to its lower electronegativity compared to oxygen. Then, we add single bonds between the carbon and each oxygen, which accounts for 4 electrons (2 electrons per single bond). This leaves us with 12 electrons to distribute.
Determining the Bonding in CO2
The remaining 12 electrons are distributed around the oxygen atoms such that each oxygen has 6 electrons (an octet) and the carbon also achieves an octet. However, simply adding these electrons as lone pairs around the oxygens would not fulfill the octet rule for carbon. To satisfy the octet rule for carbon and to accurately represent the molecule, we must consider double bonds between carbon and each oxygen. By forming double bonds (one sigma bond and one pi bond), we ensure that both carbon and oxygen atoms achieve a stable octet configuration. This results in a linear molecular geometry for CO2, with the carbon atom bonded to two oxygen atoms through double bonds.
| Molecular Component | Valence Electrons | Bonding |
|---|---|---|
| Carbon (C) | 4 | Double bonds to two O atoms |
| Oxygen (O) | 6 each | Double bond to C, with lone pairs |
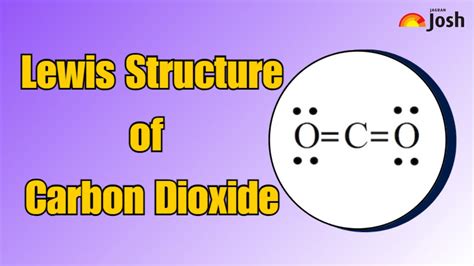
Key Points
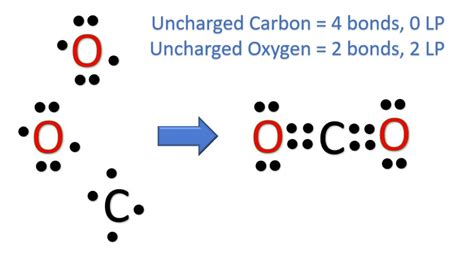
Understanding CO2 Lewis Dot Structure
- The CO2 molecule consists of one carbon and two oxygen atoms, with a total of 16 valence electrons.
- The Lewis dot structure shows double bonds between carbon and each oxygen, ensuring all atoms achieve a stable octet configuration.
- The molecule has a linear geometry due to the double bonds between carbon and oxygen atoms.
- The CO2 Lewis dot structure is crucial for understanding its chemical properties and reactivity.
- Double bonds in CO2 play a significant role in its ability to participate in chemical reactions.
Implications and Applications
The understanding of the CO2 Lewis dot structure has far-reaching implications in chemistry and related fields. It helps in predicting the reactivity of CO2, its potential to form other compounds, and its role in various biological and industrial processes. For instance, the double bonds in CO2 are crucial for its participation in photosynthesis, where it is converted into glucose by plants, releasing oxygen as a byproduct. In industrial applications, the CO2 Lewis dot structure informs the design of catalysts and reaction conditions for processes like carbon capture and utilization.
What does the CO2 Lewis dot structure reveal about its reactivity?
+The CO2 Lewis dot structure, with its double bonds between carbon and oxygen, indicates that CO2 can act as an electrophile, making it reactive towards nucleophiles. This reactivity is crucial for its role in various chemical reactions, including those involved in biological processes and industrial applications.
How does the linear geometry of CO2 influence its physical properties?
+The linear geometry of CO2, resulting from the double bonds between carbon and oxygen, contributes to its physical properties, such as its high symmetry and lack of a permanent dipole moment. This molecular shape influences its boiling point, melting point, and solubility in various solvents, which are important considerations in both biological systems and industrial processes.
In conclusion, the CO2 Lewis dot structure provides valuable insights into the molecular configuration, bonding, and reactivity of carbon dioxide. Understanding this structure is essential for grasping the chemical properties of CO2 and its roles in various processes, from photosynthesis to industrial applications. The implications of the CO2 Lewis dot structure underscore the importance of basic chemical principles in understanding complex phenomena and designing new technologies.



