F2 Lewis Structure
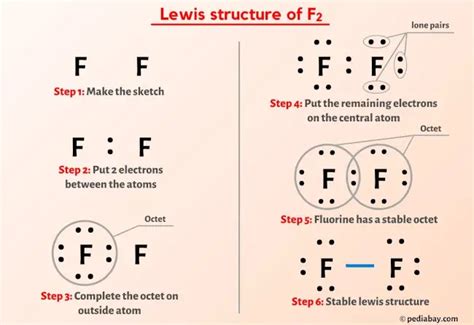
The F2 molecule, also known as fluorine gas, is a diatomic molecule composed of two fluorine atoms. To understand the structure of this molecule, we need to consider the arrangement of its electrons, which is typically represented by a Lewis structure. The Lewis structure is a useful tool in chemistry for visualizing the bonding between atoms in a molecule and the lone pairs of electrons that may exist.
Introduction to Lewis Structures

Lewis structures are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist. They are named after Gilbert N. Lewis, who introduced them in his 1916 article “The Atom and the Molecule.” Lewis structures are essential for understanding the properties and behavior of molecules, including their reactivity and shape. To draw a Lewis structure, we start with the atomic symbols of the atoms involved, and then we draw single bonds between the atoms, which represent two shared electrons. We then fill in the remaining electrons around each atom, making sure that each atom has a full outer energy level, typically eight electrons for non-hydrogen atoms, which satisfies the octet rule.
Drawing the F2 Lewis Structure
To draw the Lewis structure of F2, we start with two fluorine atoms (F) and draw a single bond between them, representing the sharing of two electrons. Each fluorine atom has seven valence electrons. When they share a pair of electrons in a covalent bond, each atom still has six electrons that are not involved in the bond. These six electrons on each fluorine atom are arranged as three lone pairs around each atom. The resulting structure shows a single bond between the two fluorine atoms, with each fluorine also having three lone pairs of electrons.
| Atom | Valence Electrons | Lone Pairs |
|---|---|---|
| Fluorine (F) | 7 | 3 |
| Oxygen (for comparison) | 6 | 2 |

Understanding the Reactivity of F2

The reactivity of F2 is largely due to its strong tendency to attract electrons. This tendency, known as electronegativity, is the highest among all elements for fluorine. As a result, F2 can readily react with other molecules to either form ions or to abstract electrons from other bonds, leading to the breaking of those bonds. This property makes fluorine gas highly useful in various chemical processes and applications, including the synthesis of fluoropolymers and the etching of silicon in the production of microelectronics.
Applications of F2
Given its high reactivity, F2 has several important applications. One of the most significant uses of fluorine is in the production of fluoropolymers, such as Teflon (polytetrafluoroethylene), which has a wide range of applications due to its non-stick properties, chemical inertness, and high thermal stability. Additionally, fluorine is used in the synthesis of hydrofluoric acid (HF), which is crucial in the etching of glass and in the semiconductor industry for the production of microchips. The use of fluorine in these and other applications underscores its importance in modern chemistry and technology.
Key Points
- The F2 molecule consists of two fluorine atoms bonded by a single covalent bond.
- Each fluorine atom in F2 has three lone pairs of electrons, which contribute to its high reactivity.
- The high electronegativity of fluorine atoms is responsible for the molecule's reactivity.
- F2 has various applications, including the synthesis of fluoropolymers and the etching of silicon in microelectronic production.
- Understanding the Lewis structure of F2 is crucial for grasping its chemical properties and reactivity.
In conclusion, the F2 molecule's Lewis structure provides valuable insights into its chemical properties and reactivity. The arrangement of electrons in F2, with a single bond between the fluorine atoms and three lone pairs on each atom, explains its tendency to react with other molecules. The applications of F2 in various industries highlight its importance in modern chemistry and technology. Through the study of Lewis structures and the properties of molecules like F2, we can deepen our understanding of chemical bonding and reactivity, ultimately leading to the development of new materials and technologies.
What is the significance of the Lewis structure in understanding molecular properties?
+The Lewis structure is significant because it allows us to visualize the arrangement of electrons in a molecule, which in turn helps us understand its chemical properties and reactivity. By looking at the Lewis structure, we can identify the types of bonds between atoms, the presence of lone pairs, and the overall molecular geometry, all of which are crucial for predicting how a molecule will behave in different chemical environments.
How does the high electronegativity of fluorine contribute to the reactivity of F2?
+The high electronegativity of fluorine means that it has a strong tendency to attract electrons towards itself. In the context of the F2 molecule, this electronegativity leads to a highly reactive molecule that can readily form bonds with other molecules by either donating or accepting electrons. This reactivity is a key factor in the many applications of fluorine and its compounds.
What are some of the most significant applications of F2 in modern technology?
+F2 has several significant applications in modern technology, including the synthesis of fluoropolymers like Teflon, which is used in non-stick coatings, and the production of hydrofluoric acid, which is essential for etching silicon wafers in the semiconductor industry. These applications take advantage of the unique properties of fluorine, such as its high reactivity and the strength of the carbon-fluorine bond, to create materials and devices with exceptional performance characteristics.
