Is Cs2 Polar Or Nonpolar
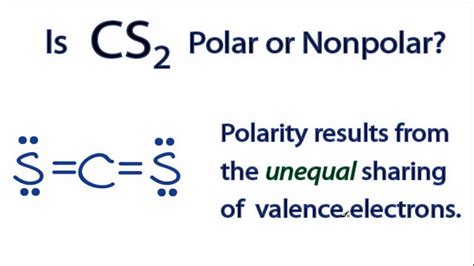
The question of whether CS2 (carbon disulfide) is polar or nonpolar is a fundamental inquiry in chemistry, particularly in understanding the properties and behaviors of molecules. To address this, we first need to understand what polarity in a molecule means. Polarity refers to the separation of electric charge within a molecule, resulting in a molecule or its chemical groups having an electric dipole moment. This separation of charge is often due to the difference in electronegativity between atoms in a covalent bond.
Understanding CS2 Molecular Structure
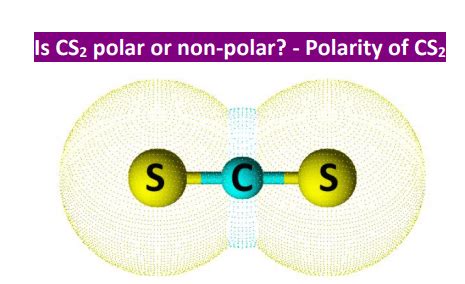
CS2 is composed of one carbon atom bonded to two sulfur atoms through covalent bonds. The molecular structure of CS2 is linear, with the carbon atom at the center and the sulfur atoms at either end. This linear geometry is crucial in determining the polarity of the molecule.
Electronegativity and Bond Polarity
The electronegativity of an atom is a measure of its ability to attract electrons in a covalent bond. Carbon has an electronegativity value of approximately 2.5, and sulfur has an electronegativity value of about 2.5 as well. Since the electronegativities of carbon and sulfur are very similar, the bonds between carbon and sulfur in CS2 are considered to be nonpolar covalent bonds.
However, even though the individual bonds are nonpolar, the overall polarity of a molecule also depends on its shape. In the case of CS2, despite the nonpolar nature of the C-S bonds, if the molecule were bent or had a different shape, it could potentially be polar due to the asymmetrical distribution of electron density. But, given its linear shape, the two C-S bond dipoles (which are very small due to the similar electronegativities) cancel each other out.
| Atom | Electronegativity |
|---|---|
| Carbon (C) | 2.5 |
| Sulfur (S) | 2.5 |
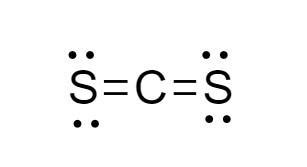
Implications of Nonpolarity

The nonpolar nature of CS2 has significant implications for its physical and chemical properties. Nonpolar molecules tend to have lower boiling points compared to polar molecules of similar molecular weight because they do not form hydrogen bonds with each other. CS2, with its nonpolar character, exhibits properties such as being insoluble in water (a polar solvent) but soluble in nonpolar solvents like chloroform or benzene.
Key Points
- CS2 has a linear molecular geometry.
- The carbon and sulfur atoms have similar electronegativities, making the C-S bonds nonpolar.
- The linear shape of CS2 means that any slight dipoles in the C-S bonds cancel out, resulting in no net dipole moment.
- CS2 is insoluble in water but soluble in nonpolar solvents, demonstrating its nonpolar nature.
- The nonpolarity of CS2 influences its physical properties, such as its boiling point.
In conclusion, the polarity of a molecule is determined by both the electronegativity differences in its bonds and its overall shape. For CS2, despite the potential for slight polarity in the individual bonds due to electronegativity differences, its linear geometry and the similarity in electronegativities between carbon and sulfur result in a nonpolar molecule.
What determines the polarity of a molecule?
+The polarity of a molecule is determined by the difference in electronegativity between the atoms in a covalent bond and the shape of the molecule. A significant difference in electronegativity can lead to polar bonds, and if the molecule's shape is asymmetrical, it can result in a polar molecule.
Is CS2 soluble in water?
+No, CS2 is not soluble in water due to its nonpolar nature. It is, however, soluble in nonpolar solvents like chloroform or benzene.
What is the boiling point of CS2, and how does its nonpolarity affect it?
+The boiling point of CS2 is 46.24°C. Its nonpolarity results in lower boiling points compared to polar molecules of similar molecular weight because nonpolar molecules do not form hydrogen bonds, which require more energy to break.
Meta Description: Explore the polarity of CS2, understanding how its linear geometry and the electronegativities of its constituent atoms influence its chemical properties and behavior.