Is Salt A Compound
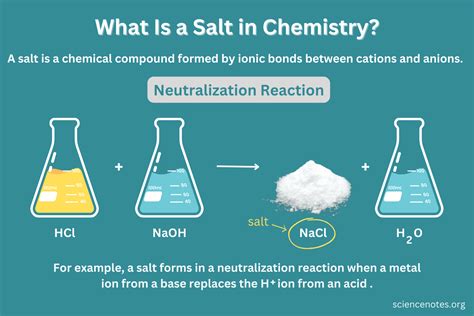
Salt, commonly referred to as table salt, is a crystalline mineral composed primarily of sodium chloride (NaCl). The question of whether salt is a compound can be answered by examining its chemical composition and structure. A compound, in chemical terms, is a substance formed when two or more different elements are chemically bonded together. In the case of salt, it consists of two elements: sodium (Na) and chlorine (Cl), which are chemically bonded in a 1:1 ratio.
Chemical Composition of Salt

The chemical formula for salt, NaCl, indicates that it is indeed a compound because it is made up of two different elements. The bonding between sodium and chlorine in salt is ionic, meaning that one or more electrons are transferred between the atoms, leading to the formation of ions with opposite charges. Sodium loses an electron to become a positively charged sodium ion (Na+), while chlorine gains an electron to become a negatively charged chloride ion (Cl-). The electrostatic attraction between the positively charged sodium ions and the negatively charged chloride ions holds them together in a rigid framework, forming the crystalline structure of salt.
Characteristics of Compounds
To further classify salt as a compound, we can look at the characteristics that define compounds. These include having a fixed composition, being chemically bonded, and exhibiting properties different from those of their constituent elements. Salt meets these criteria: it has a fixed composition (always one sodium atom for every chlorine atom), the elements are chemically bonded (through ionic bonds), and its properties are distinct from those of pure sodium and chlorine. For example, while pure sodium is highly reactive and pure chlorine is a toxic gas, salt is relatively inert and safe for human consumption in moderate amounts.
| Element | Symbol | Atomic Number |
|---|---|---|
| Sodium | Na | 11 |
| Chlorine | Cl | 17 |

Key Points
- Salt, or sodium chloride (NaCl), is a compound consisting of two elements: sodium and chlorine.
- The chemical bonding in salt is ionic, with sodium ions (Na+) and chloride ions (Cl-) attracting each other.
- Salt meets the criteria for a compound: it has a fixed composition, its elements are chemically bonded, and it exhibits properties different from its constituent elements.
- The properties of salt, such as its taste, texture, and reactivity, are distinct from those of pure sodium and chlorine.
- Understanding salt as a compound is essential for its application in various fields, including food, medicine, and industry.
Given the information above, it's clear that salt is indeed a compound. Its composition of sodium and chlorine, held together by ionic bonds, and its properties that are unique compared to its constituent elements, fulfill the definition of a compound. This understanding is not only interesting from a chemical standpoint but also has practical implications for how we use and interact with salt in our daily lives.
Practical Applications of Salt as a Compound

The recognition of salt as a compound informs its various applications. In cooking, the balance of sodium and chlorine contributes to the flavor enhancement properties of salt. In medicine, understanding the chemical composition of salt is crucial for managing conditions related to sodium and chloride imbalances in the body. Industrially, the compound nature of salt makes it useful in the manufacture of other chemicals, such as sodium carbonate and chlorine gas, which have applications in glassmaking, paper production, and water treatment, among others.
Evidence-Based Analysis
Evidence from chemistry supports the classification of salt as a compound. Chemical analyses, including spectroscopy and chromatography, can identify the presence of sodium and chlorine in salt and confirm their 1:1 ratio. Additionally, experiments demonstrating the formation of salt from sodium and chlorine, such as the reaction of sodium metal with chlorine gas, provide empirical evidence for the compound nature of salt.
What is the chemical formula for salt, and what does it indicate about its composition?
+The chemical formula for salt is NaCl, indicating it is composed of sodium (Na) and chlorine (Cl) in a 1:1 ratio, classifying it as a compound.
What type of bond holds the sodium and chlorine ions together in salt?
+The sodium and chlorine ions in salt are held together by ionic bonds, which form when electrons are transferred between the atoms, resulting in the attraction between positively charged sodium ions and negatively charged chloride ions.
How does understanding salt as a compound impact its practical applications?
+Recognizing salt as a compound is crucial for its applications in cooking, medicine, and industry, as it informs how salt interacts with other substances and contributes to various processes and products.
Meta Description: Discover the composition and properties of salt, understanding why it is classified as a compound and exploring its significance in various applications.



