Khp Molar Mass
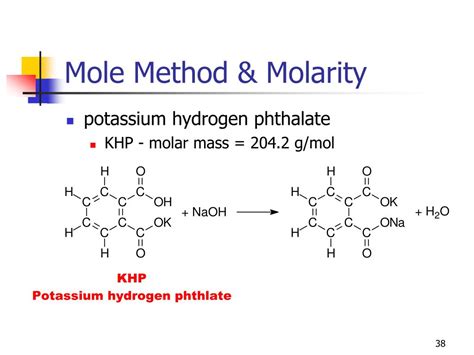
The molar mass of a compound is a fundamental concept in chemistry, representing the mass of one mole of that substance. For potassium hydrogen phthalate, commonly abbreviated as KHP, its molar mass is a crucial piece of information for chemists and researchers. KHP is widely used as a primary standard in acid-base titrations due to its high purity, stability, and well-defined composition. The chemical formula for potassium hydrogen phthalate is KC₈H₅O₄.
Calculation of Molar Mass of KHP

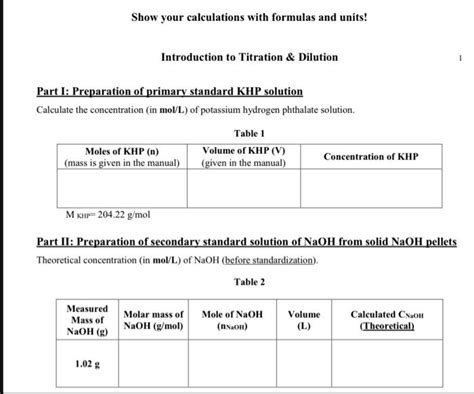
To calculate the molar mass of KHP, we sum the atomic masses of all the atoms in one molecule of the compound. The atomic masses (rounded to the nearest whole number for simplicity) are approximately: Potassium (K) = 39 g/mol, Carbon © = 12 g/mol, Hydrogen (H) = 1 g/mol, and Oxygen (O) = 16 g/mol.
Using the formula KC₈H₅O₄, we calculate the molar mass as follows: - Potassium (K): 1 * 39 = 39 g/mol - Carbon (C): 8 * 12 = 96 g/mol - Hydrogen (H): 5 * 1 = 5 g/mol - Oxygen (O): 4 * 16 = 64 g/mol Adding these values together: 39 + 96 + 5 + 64 = 204 g/mol.
Precision in Atomic Masses
It’s worth noting that the actual atomic masses used in precise calculations are not rounded and can be found on the periodic table. For example, the atomic mass of potassium is more precisely 39.0983 g/mol, carbon is 12.0107 g/mol, hydrogen is 1.00794 g/mol, and oxygen is 15.9994 g/mol. Using these precise values, the molar mass of KHP can be calculated with higher accuracy.
Recalculating with precise atomic masses: - Potassium (K): 1 * 39.0983 = 39.0983 g/mol - Carbon (C): 8 * 12.0107 = 96.0856 g/mol - Hydrogen (H): 5 * 1.00794 = 5.0397 g/mol - Oxygen (O): 4 * 15.9994 = 63.9976 g/mol Total: 39.0983 + 96.0856 + 5.0397 + 63.9976 = 204.2212 g/mol.
| Element | Atomic Mass (g/mol) | Number in KHP | Total Mass Contribution |
|---|---|---|---|
| Potassium (K) | 39.0983 | 1 | 39.0983 |
| Carbon (C) | 12.0107 | 8 | 96.0856 |
| Hydrogen (H) | 1.00794 | 5 | 5.0397 |
| Oxygen (O) | 15.9994 | 4 | 63.9976 |
| Total | 204.2212 |
Key Points
- The molar mass of KHP (KC₈H₅O₄) is calculated by summing the atomic masses of its constituent elements.
- Using approximate atomic masses, the molar mass of KHP is approximately 204 g/mol.
- With precise atomic masses, the molar mass of KHP is more accurately calculated as 204.2212 g/mol.
- KHP's precise molar mass is essential for its use as a primary standard in acid-base titrations.
- Understanding the molar mass of compounds is fundamental in chemistry for calculating quantities and concentrations in reactions.
In conclusion, the molar mass of potassium hydrogen phthalate (KHP) is a critical value for its application in chemical analyses. The precise calculation of its molar mass, considering the exact atomic masses of its constituent elements, ensures the accuracy and reliability of chemical reactions and analyses where KHP is utilized.
What is the primary use of KHP in chemistry?
+KHP is primarily used as a standard in acid-base titrations due to its high purity and well-defined composition.
Why is the precise molar mass of KHP important?
+The precise molar mass of KHP is crucial for accurate calculations in chemical analyses, particularly in titration processes.
How is the molar mass of a compound calculated?
+The molar mass of a compound is calculated by summing the atomic masses of all the atoms in one molecule of the compound.