Kinetic Molecular Theory Of Gases
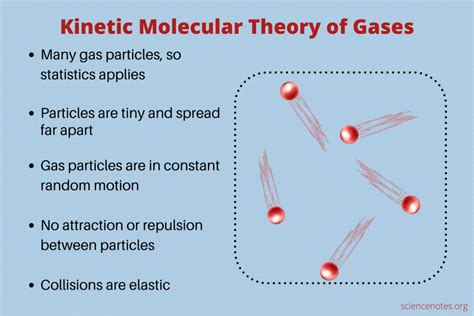
The kinetic molecular theory of gases is a fundamental concept in physics and chemistry that explains the behavior of gases. It is based on the idea that gases are composed of tiny particles, called molecules, which are in constant motion. The theory was developed in the 19th century by scientists such as August Krönig, Rudolf Clausius, and Ludwig Boltzmann, and it has been widely accepted as a fundamental principle of physics and chemistry. The kinetic molecular theory of gases is a complex and multifaceted concept that has far-reaching implications for our understanding of the behavior of gases and their interactions with other substances.
The kinetic molecular theory of gases is based on several key postulates. Firstly, it assumes that gases are composed of tiny particles, called molecules, which are in constant motion. These molecules are assumed to be point particles, meaning that they have no size or shape, and they are in constant random motion. Secondly, the theory assumes that the molecules of a gas are in constant collision with each other and with the walls of their container. These collisions are perfectly elastic, meaning that the molecules bounce off each other and the walls of the container without losing any energy. Finally, the theory assumes that the molecules of a gas are in a state of thermal equilibrium, meaning that they have the same average kinetic energy.
Key Points
- The kinetic molecular theory of gases is based on the idea that gases are composed of tiny particles, called molecules, which are in constant motion.
- The theory assumes that the molecules of a gas are in constant collision with each other and with the walls of their container.
- The molecules of a gas are in a state of thermal equilibrium, meaning that they have the same average kinetic energy.
- The kinetic molecular theory of gases is a fundamental concept in physics and chemistry that explains the behavior of gases.
- The theory has far-reaching implications for our understanding of the behavior of gases and their interactions with other substances.
Postulates of the Kinetic Molecular Theory of Gases
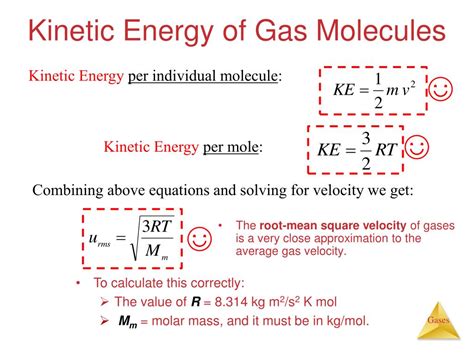
The kinetic molecular theory of gases is based on several key postulates. These postulates are the foundation of the theory and are used to derive the various equations and formulas that describe the behavior of gases. The postulates of the kinetic molecular theory of gases are as follows:
Postulate 1: Gases are Composed of Tiny Particles
The first postulate of the kinetic molecular theory of gases is that gases are composed of tiny particles, called molecules. These molecules are assumed to be point particles, meaning that they have no size or shape. The molecules of a gas are in constant random motion, and they are free to move in any direction.
Postulate 2: The Molecules of a Gas are in Constant Collision
The second postulate of the kinetic molecular theory of gases is that the molecules of a gas are in constant collision with each other and with the walls of their container. These collisions are perfectly elastic, meaning that the molecules bounce off each other and the walls of the container without losing any energy.
Postulate 3: The Molecules of a Gas are in a State of Thermal Equilibrium
The third postulate of the kinetic molecular theory of gases is that the molecules of a gas are in a state of thermal equilibrium. This means that the molecules have the same average kinetic energy, and they are distributed randomly throughout the container.
| Postulate | Description |
|---|---|
| 1 | Gases are composed of tiny particles, called molecules. |
| 2 | The molecules of a gas are in constant collision with each other and with the walls of their container. |
| 3 | The molecules of a gas are in a state of thermal equilibrium, meaning that they have the same average kinetic energy. |
Implications of the Kinetic Molecular Theory of Gases
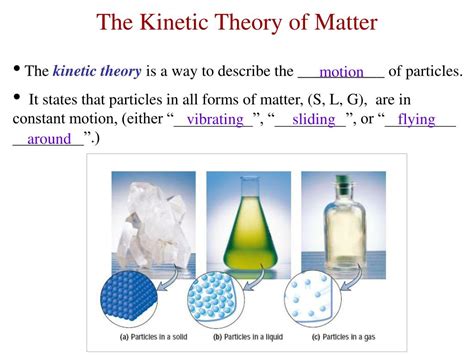
The kinetic molecular theory of gases has far-reaching implications for our understanding of the behavior of gases and their interactions with other substances. The theory explains many of the properties of gases, including their pressure, volume, and temperature. It also provides a framework for understanding the behavior of gases in different situations, such as in a container or in the atmosphere.
Pressure of a Gas
The pressure of a gas is the result of the collisions between the molecules of the gas and the walls of their container. The more frequent and energetic the collisions, the higher the pressure of the gas. The pressure of a gas is directly proportional to the average kinetic energy of the molecules and inversely proportional to the volume of the container.
Volume of a Gas
The volume of a gas is the space occupied by the molecules of the gas. The volume of a gas is directly proportional to the number of molecules and the average distance between them. The volume of a gas can be affected by changes in pressure and temperature.
Temperature of a Gas
The temperature of a gas is a measure of the average kinetic energy of the molecules. The higher the temperature, the more energetic the molecules and the more frequent and energetic the collisions. The temperature of a gas is directly proportional to the average kinetic energy of the molecules and inversely proportional to the volume of the container.
What is the kinetic molecular theory of gases?
+The kinetic molecular theory of gases is a fundamental concept in physics and chemistry that explains the behavior of gases. It is based on the idea that gases are composed of tiny particles, called molecules, which are in constant motion.
What are the postulates of the kinetic molecular theory of gases?
+The postulates of the kinetic molecular theory of gases are: (1) gases are composed of tiny particles, called molecules, (2) the molecules of a gas are in constant collision with each other and with the walls of their container, and (3) the molecules of a gas are in a state of thermal equilibrium.
What are the implications of the kinetic molecular theory of gases?
+The kinetic molecular theory of gases has far-reaching implications for our understanding of the behavior of gases and their interactions with other substances. It explains many of the properties of gases, including their pressure, volume, and temperature.