Molecular Weight Of Air
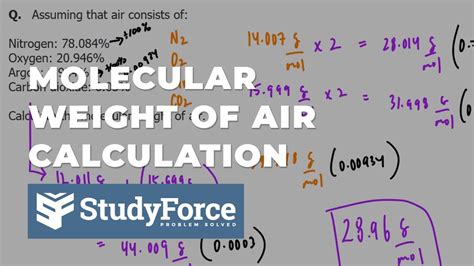
The molecular weight of air is a fundamental concept in physics and chemistry, particularly in the fields of aerodynamics, thermodynamics, and environmental science. Air is a mixture of gases, primarily consisting of nitrogen (N2), oxygen (O2), argon (Ar), and trace amounts of other gases. The molecular weight of air is calculated by determining the average molecular weight of its constituent gases, taking into account their respective proportions.
To calculate the molecular weight of air, we need to know the molecular weights of its primary components. The molecular weight of nitrogen (N2) is approximately 28.01 g/mol, oxygen (O2) is 31.99 g/mol, and argon (Ar) is 39.95 g/mol. Other gases present in air, such as carbon dioxide (CO2), neon (Ne), and helium (He), have molecular weights of 44.01 g/mol, 20.18 g/mol, and 4.003 g/mol, respectively. The proportion of these gases in dry air is approximately 78.08% nitrogen, 20.95% oxygen, 0.93% argon, and 0.04% carbon dioxide, with trace amounts of other gases.
Key Points
- The molecular weight of air is approximately 28.97 g/mol, calculated based on the average molecular weights of its constituent gases.
- Nitrogen (N2) constitutes about 78.08% of dry air, with a molecular weight of 28.01 g/mol.
- Oxygen (O2) makes up about 20.95% of dry air, with a molecular weight of 31.99 g/mol.
- Argon (Ar) is present in about 0.93% of dry air, with a molecular weight of 39.95 g/mol.
- Other gases, including carbon dioxide (CO2), neon (Ne), and helium (He), are present in trace amounts and contribute to the overall molecular weight of air.
Calculation of Molecular Weight of Air
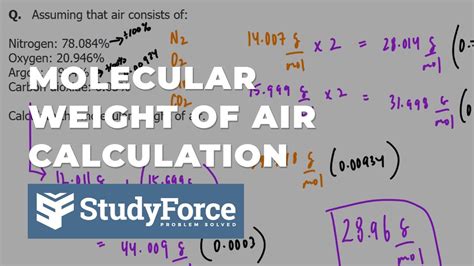
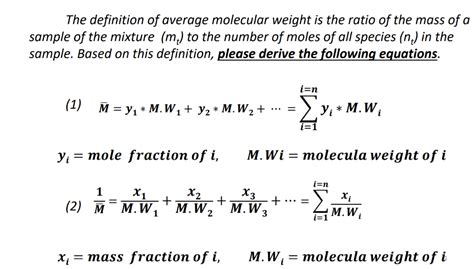
The calculation of the molecular weight of air involves determining the weighted average of the molecular weights of its constituent gases. This is done by multiplying the molecular weight of each gas by its proportion in the air and then summing these products. The formula for calculating the molecular weight of air (MWA) is given by:
MWA = (0.7808 * 28.01) + (0.2095 * 31.99) + (0.0093 * 39.95) + (0.0004 * 44.01) +...
where the numbers in parentheses represent the proportions of nitrogen, oxygen, argon, and carbon dioxide in dry air, respectively. By performing the calculation, we find that the molecular weight of air is approximately 28.97 g/mol.
Variations in Molecular Weight of Air
The molecular weight of air can vary slightly depending on factors such as temperature, humidity, and altitude. At higher temperatures, the molecular weight of air decreases due to the increased proportion of lighter gases. Conversely, at lower temperatures, the molecular weight increases due to the increased proportion of heavier gases. Humidity also affects the molecular weight of air, as water vapor (H2O) has a molecular weight of 18.02 g/mol, which is significantly lower than the average molecular weight of dry air.
| Gas | Molecular Weight (g/mol) | Proportion in Dry Air |
|---|---|---|
| Nitrogen (N2) | 28.01 | 78.08% |
| Oxygen (O2) | 31.99 | 20.95% |
| Argon (Ar) | 39.95 | 0.93% |
| Carbon Dioxide (CO2) | 44.01 | 0.04% |
Applications of Molecular Weight of Air
The molecular weight of air has numerous applications in various fields, including aerodynamics, thermodynamics, and environmental science. In aerodynamics, the molecular weight of air is used to calculate air density, which is essential for predicting the behavior of aircraft and wind turbines. In thermodynamics, the molecular weight of air is used to calculate the specific heat capacity and thermal conductivity of air, which are critical parameters in the design of heating, ventilation, and air conditioning (HVAC) systems.
In environmental science, the molecular weight of air is used to study the behavior of pollutants and greenhouse gases in the atmosphere. By understanding the molecular weight and composition of air, scientists can predict the transport and fate of pollutants and develop strategies for mitigating their impacts on the environment.
Conclusion
In conclusion, the molecular weight of air is a fundamental concept that has numerous applications in various fields. By understanding the composition and molecular weight of air, scientists and engineers can predict its behavior under different conditions and design systems that interact with air. The molecular weight of air is approximately 28.97 g/mol, calculated based on the average molecular weights of its constituent gases. Variations in temperature, humidity, and altitude can affect the molecular weight of air, and understanding these factors is essential for predicting its behavior in different environments.
What is the molecular weight of air?
+The molecular weight of air is approximately 28.97 g/mol, calculated based on the average molecular weights of its constituent gases.
What are the primary components of air?
+The primary components of air are nitrogen (N2), oxygen (O2), argon (Ar), and trace amounts of other gases, including carbon dioxide (CO2), neon (Ne), and helium (He).
How does temperature affect the molecular weight of air?
+Temperature affects the molecular weight of air by changing the proportion of lighter and heavier gases. At higher temperatures, the molecular weight of air decreases due to the increased proportion of lighter gases.
Meta description suggestion: “Discover the molecular weight of air, its composition, and variations with temperature, humidity, and altitude. Learn about its applications in aerodynamics, thermodynamics, and environmental science.” (149 characters)