Periodic Table Of Elements With Valence Electrons

The Periodic Table of Elements is a fundamental tool in chemistry, providing a comprehensive framework for understanding the properties and behavior of elements. One crucial aspect of the Periodic Table is the concept of valence electrons, which play a pivotal role in determining an element's chemical reactivity and bonding capabilities. In this article, we will delve into the world of valence electrons and explore their significance in the context of the Periodic Table.
Introduction to Valence Electrons

Valence electrons are the electrons in the outermost energy level of an atom, which is also known as the valence shell. These electrons are responsible for participating in chemical bonding and reactions, as they are the most loosely bound to the nucleus and are therefore more accessible to other atoms. The number of valence electrons an atom has determines its chemical properties and reactivity.
Valence Electrons and the Periodic Table
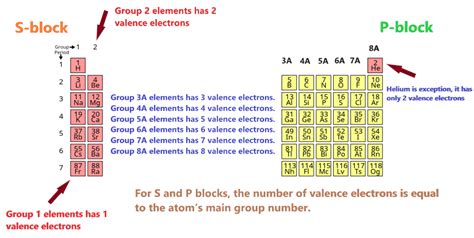
The Periodic Table is arranged in a way that elements with similar chemical properties and valence electron configurations are grouped together. The horizontal rows of the Periodic Table are called periods, and the vertical columns are called groups or families. Elements in the same group have the same number of valence electrons, which results in similar chemical properties. For example, all the elements in Group 1 (the alkali metals) have one valence electron, while all the elements in Group 17 (the halogens) have seven valence electrons.
| Group Number | Valence Electrons | Element Examples |
|---|---|---|
| 1 (Alkali Metals) | 1 | Lithium (Li), Sodium (Na), Potassium (K) |
| 2 (Alkaline Earth Metals) | 2 | Magnesium (Mg), Calcium (Ca), Strontium (Sr) |
| 17 (Halogens) | 7 | Fluorine (F), Chlorine (Cl), Bromine (Br) |
| 18 (Noble Gases) | 8 (full outer shell) | Helium (He), Neon (Ne), Argon (Ar) |
Key Points

Key Points
- Valence electrons are the electrons in the outermost energy level of an atom and determine an element’s chemical reactivity.
- The Periodic Table is arranged to group elements with similar valence electron configurations together, resulting in similar chemical properties.
- Elements in the same group have the same number of valence electrons, while elements in the same period have the same number of electron shells.
- The number of valence electrons an atom has determines its position in the Periodic Table and its chemical behavior.
- Understanding valence electrons is crucial for predicting chemical reactions and properties, such as electronegativity, electron affinity, and ionization energy.
Valence Electrons and Chemical Bonding
Valence electrons play a crucial role in chemical bonding, as they are responsible for forming bonds between atoms. The number of valence electrons an atom has determines the types of bonds it can form and the number of bonds it can form. For example, atoms with a full outer shell (noble gases) are unreactive, as they have no valence electrons available for bonding. On the other hand, atoms with incomplete outer shells (such as the alkali metals and halogens) are highly reactive, as they tend to gain or lose electrons to achieve a full outer shell.
The type of bond formed between atoms also depends on the number of valence electrons. For example, covalent bonds are formed when two or more atoms share one or more pairs of valence electrons, while ionic bonds are formed when one or more electrons are transferred from one atom to another, resulting in the formation of ions with opposite charges.
Implications of Valence Electrons in Chemistry
The concept of valence electrons has far-reaching implications in chemistry, as it allows for the prediction of chemical properties and reactivity. By understanding the valence electron configuration of an element, chemists can predict its chemical behavior, including its tendency to form bonds, its reactivity, and its chemical properties. This knowledge is essential for a wide range of applications, from the development of new materials and pharmaceuticals to the understanding of biological processes and environmental phenomena.
What is the significance of valence electrons in the Periodic Table?
+Valence electrons determine an element's chemical properties and reactivity, and the Periodic Table is arranged to group elements with similar valence electron configurations together.
How do valence electrons affect chemical bonding?
+Valence electrons are responsible for forming bonds between atoms, and the number of valence electrons an atom has determines the types of bonds it can form and the number of bonds it can form.
What is the relationship between valence electrons and the Periodic Table groups?
+Elements in the same group have the same number of valence electrons, which results in similar chemical properties.
In conclusion, valence electrons play a vital role in understanding the chemical properties and behavior of elements, and the Periodic Table provides a comprehensive framework for organizing and predicting these properties. By grasping the concept of valence electrons and their significance in the Periodic Table, chemists and researchers can unlock a deeper understanding of the chemical world and develop new materials, technologies, and applications that transform our lives.
Related Terms:
- Periodic table with valency
- Valency of all 118 elements
- How to find valence electrons
- Valency table Class 9



