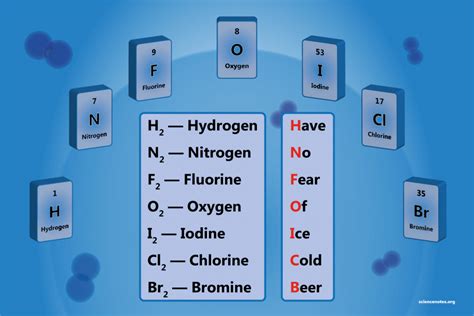
Seven Diatomic Elements
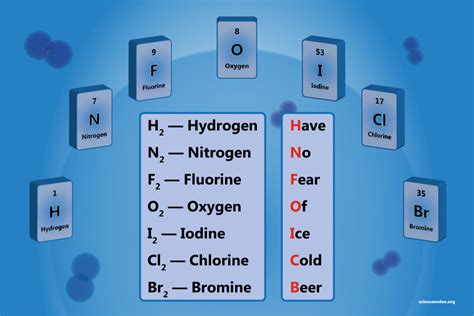
The periodic table is a comprehensive chart that organizes elements based on their atomic structure and chemical properties. Among these elements, diatomic molecules are a unique group that consists of two atoms bonded together. There are seven diatomic elements, which are substances that exist as diatomic molecules in their natural state. These elements are crucial in understanding chemistry and are widely used in various industrial applications. In this article, we will delve into the world of diatomic elements, exploring their properties, uses, and significance in the periodic table.
Key Points
- The seven diatomic elements are Hydrogen (H₂), Nitrogen (N₂), Oxygen (O₂), Fluorine (F₂), Chlorine (Cl₂), Bromine (Br₂), and Iodine (I₂)
- Diatomic elements are highly reactive due to their molecular structure
- These elements are essential in various industrial processes, such as the production of fertilizers, plastics, and pharmaceuticals
- Diatomic elements also play a crucial role in the Earth's atmosphere, with Oxygen and Nitrogen making up approximately 99% of the air we breathe
- The unique properties of diatomic elements make them vital in research and development, particularly in the fields of chemistry and physics
Properties and Characteristics of Diatomic Elements

Diatomic elements are characterized by their molecular structure, which consists of two atoms bonded together through a covalent bond. This bond is strong and stable, resulting in a molecule with unique properties. For instance, the bond length and bond energy of diatomic molecules vary greatly, influencing their reactivity and stability. The physical properties of diatomic elements, such as their melting and boiling points, also differ significantly from those of other elements.
Chemical Reactivity of Diatomic Elements
The chemical reactivity of diatomic elements is a critical aspect of their properties. Due to their molecular structure, these elements are highly reactive and tend to form compounds with other elements. For example, Hydrogen (H₂) is highly flammable and reacts violently with Oxygen (O₂) to form water (H₂O). Similarly, Nitrogen (N₂) and Oxygen (O₂) react to form Nitric Oxide (NO), a crucial component in the production of fertilizers and explosives.
| Diatomic Element | Molecular Structure | Bond Length (pm) | Bond Energy (kJ/mol) |
|---|---|---|---|
| Hydrogen (H₂) | H-H | 74 | 436 |
| Nitrogen (N₂) | N-N | 110 | 945 |
| Oxygen (O₂) | O-O | 121 | 498 |
| Fluorine (F₂) | F-F | 142 | 159 |
| Chlorine (Cl₂) | Cl-Cl | 199 | 243 |
| Bromine (Br₂) | Br-Br | 228 | 193 |
| Iodine (I₂) | I-I | 267 | 151 |
Industrial Applications of Diatomic Elements
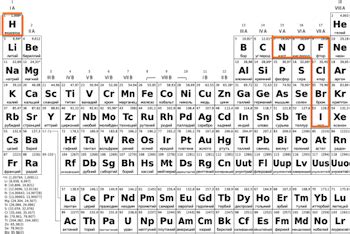
The seven diatomic elements have numerous industrial applications due to their unique properties and reactivity. For instance, Nitrogen (N₂) and Oxygen (O₂) are used in the production of fertilizers, while Fluorine (F₂) is used in the manufacture of plastics and pharmaceuticals. Chlorine (Cl₂) is used as a disinfectant and in the production of paper and textiles. Bromine (Br₂) is used in the production of dyes and pharmaceuticals, while Iodine (I₂) is used in the production of catalysts and pharmaceuticals.
Environmental Significance of Diatomic Elements
Diatomic elements also play a crucial role in the Earth’s atmosphere. Oxygen (O₂) and Nitrogen (N₂) make up approximately 99% of the air we breathe, with Oxygen being essential for human respiration and Nitrogen being a critical component of amino acids and nucleotides. The balance of these elements in the atmosphere is vital for maintaining the health of our planet and supporting life on Earth.
What are the seven diatomic elements?
+The seven diatomic elements are Hydrogen (H₂), Nitrogen (N₂), Oxygen (O₂), Fluorine (F₂), Chlorine (Cl₂), Bromine (Br₂), and Iodine (I₂).
What are the unique properties of diatomic elements?
+Diatomic elements have a unique molecular structure, consisting of two atoms bonded together through a covalent bond. This bond is strong and stable, resulting in a molecule with unique properties, such as varying bond lengths and bond energies.
What are the industrial applications of diatomic elements?
+The seven diatomic elements have numerous industrial applications, including the production of fertilizers, plastics, pharmaceuticals, and dyes. They are also used as disinfectants, in the production of paper and textiles, and as catalysts in various chemical reactions.
In conclusion, the seven diatomic elements are a unique group of substances that exist as diatomic molecules in their natural state. Their properties, reactivity, and industrial applications make them essential in various fields, from chemistry and physics to environmental science and industry. Understanding the molecular structure and chemical reactivity of these elements is crucial in developing new technologies and applications, and their significance in the Earth’s atmosphere makes them vital for maintaining the health of our planet.