Sodium Hydroxide Formula

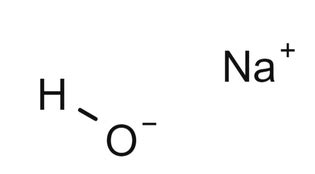
Sodium hydroxide, commonly known as lye or caustic soda, is a highly alkaline substance with a wide range of industrial, medical, and household applications. The sodium hydroxide formula, NaOH, represents its chemical composition, consisting of one sodium (Na) atom, one oxygen (O) atom, and one hydrogen (H) atom. This strong base is highly soluble in water, releasing hydroxide ions (OH-) and sodium ions (Na+) upon dissolution. The chemical properties and reactivity of sodium hydroxide are fundamental to understanding its uses and handling requirements.
Chemical Structure and Properties

The chemical structure of sodium hydroxide can be represented as a simple ionic compound, where the sodium ion (Na+) is bonded to the hydroxide ion (OH-). This ionic bond is highly polar, contributing to the compound’s high solubility in water and its strong alkaline properties. Sodium hydroxide is a white, odorless solid at room temperature, highly caustic and corrosive, requiring careful handling to avoid skin and eye irritation or more severe injuries. The melting point of sodium hydroxide is approximately 318°C (604°F), and it boils at around 1388°C (2530°F), though it typically decomposes before reaching its boiling point.
Key Points
- Sodium hydroxide's chemical formula is NaOH, indicating its composition of sodium, oxygen, and hydrogen.
- It is a highly alkaline substance, soluble in water, and releases hydroxide and sodium ions in solution.
- The compound has a wide range of applications, including in the manufacture of soap, paper, and textiles.
- Sodium hydroxide is highly caustic and requires careful handling to prevent injuries.
- Its chemical properties, such as high solubility and strong alkalinity, are critical for its industrial and household uses.
Sodium Hydroxide Production and Applications
Sodium hydroxide is produced on a large scale through the electrolysis of sodium chloride (NaCl) solutions, known as the chlor-alkali process. This process involves the electrolytic decomposition of sodium chloride into chlorine gas (Cl2) and sodium hydroxide. The production of sodium hydroxide is a significant industrial activity, given its diverse applications. It is used in the manufacture of soap, detergents, and other cleaning products, where its ability to form surfactants and emulsify oils is crucial. Additionally, sodium hydroxide is used in the paper industry for pulping wood, in the textile industry for treating and processing fabrics, and in various chemical syntheses, including the production of rayon and spandex.
| Application Area | Role of Sodium Hydroxide |
|---|---|
| Soap and Detergent Manufacturing | Forms surfactants, emulsifies oils |
| Paper Industry | Pulping wood, bleaching |
| Textile Industry | Treating and processing fabrics |
| Chemical Syntheses | Production of rayon, spandex, and other chemicals |
Safety Considerations and Handling
Given its highly caustic nature, sodium hydroxide poses significant safety risks if not handled properly. It can cause severe burns upon contact with skin and eyes, and inhalation of its dust can lead to respiratory irritation. Therefore, handling sodium hydroxide requires wearing protective clothing, including gloves, goggles, and a face mask. When dissolved in water, the solution should be handled with care, as it can still cause chemical burns. In case of accidental exposure, immediate rinsing with plenty of water is crucial, followed by medical attention if necessary.
Environmental Impact
The production and disposal of sodium hydroxide can have environmental implications. The chlor-alkali process, for instance, involves the release of chlorine gas, which is toxic and can contribute to environmental pollution if not properly managed. Moreover, the disposal of sodium hydroxide and its by-products requires careful consideration to prevent contamination of water sources and soil. Efforts to minimize waste and implement sustainable production practices are essential to reducing the environmental footprint of sodium hydroxide production.
What are the primary uses of sodium hydroxide?
+Sodium hydroxide is primarily used in the manufacture of soap, paper, and textiles, as well as in various chemical syntheses.
Why is sodium hydroxide highly soluble in water?
+Sodium hydroxide's high solubility in water is due to its ionic nature, which allows it to dissociate into sodium and hydroxide ions in aqueous solutions.
What safety precautions should be taken when handling sodium hydroxide?
+Handling sodium hydroxide requires wearing protective clothing, including gloves, goggles, and a face mask, and avoiding inhalation of its dust. In case of accidental exposure, immediate rinsing with water and medical attention if necessary are crucial.
In conclusion, sodium hydroxide, with its formula NaOH, is a fundamental chemical compound with a wide array of applications across various industries. Its unique chemical properties, including high solubility and strong alkalinity, make it an essential component in the production of numerous products. However, its handling and production also come with significant safety and environmental considerations, necessitating careful management and adherence to best practices to minimize risks and environmental impact.



