Where Is An Electron Found
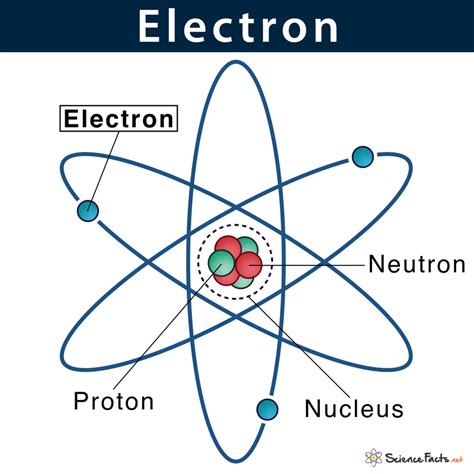
An electron is a subatomic particle that plays a crucial role in the structure and properties of atoms. To understand where an electron is found, it's essential to delve into the atomic structure. Atoms are the building blocks of matter, and they consist of three main components: protons, neutrons, and electrons. Protons and neutrons are found in the nucleus, which is the central part of the atom, while electrons are located outside the nucleus.
The electrons in an atom are arranged in a specific pattern, which is described by the electron configuration. The electron configuration is the distribution of electrons in an atom's orbitals, which are regions around the nucleus where electrons are likely to be found. The orbitals are divided into different energy levels or shells, and each shell can hold a specific number of electrons. The first shell, also known as the 1s orbital, can hold up to two electrons, while the second shell, which includes the 2s and 2p orbitals, can hold up to eight electrons.
Electron Location in Atoms

In an atom, electrons are found in the electron cloud, which is the region outside the nucleus. The electron cloud is not a fixed location but rather a probability distribution of where electrons are likely to be found. The shape and size of the electron cloud depend on the energy level and the type of orbital. For example, the 1s orbital is spherical in shape and is closest to the nucleus, while the 2p orbitals are dumbbell-shaped and are farther away from the nucleus.
Energy Levels and Electron Position
The energy levels or shells in an atom are designated by the principal quantum number (n), which is an integer that starts from 1 and increases as you move away from the nucleus. The first energy level (n=1) is the closest to the nucleus and can hold up to two electrons. The second energy level (n=2) can hold up to eight electrons, and the third energy level (n=3) can hold up to 18 electrons. Electrons in higher energy levels are farther away from the nucleus and have more energy than electrons in lower energy levels.
| Energy Level | Electron Capacity | Description |
|---|---|---|
| 1 (n=1) | 2 electrons | Closest to the nucleus, spherical shape |
| 2 (n=2) | 8 electrons | Includes 2s and 2p orbitals, farther from the nucleus |
| 3 (n=3) | 18 electrons | Includes 3s, 3p, and 3d orbitals, even farther from the nucleus |

The position of an electron in an atom is described by its orbital, which is a mathematical function that describes the probability of finding an electron within a particular region of space. The orbital is characterized by three quantum numbers: the principal quantum number (n), the azimuthal quantum number (l), and the magnetic quantum number (m). These quantum numbers determine the energy, shape, and orientation of the orbital.
Electron Movement and Spin

Electrons in an atom are not stationary but are constantly moving around the nucleus. The movement of electrons is described by the Heisenberg uncertainty principle, which states that it is impossible to know both the position and momentum of an electron with infinite precision. The spin of an electron is a fundamental property that determines its intrinsic angular momentum. Electrons can have either a +1⁄2 or -1⁄2 spin, which is a measure of their intrinsic angular momentum.
Key Points
- Electrons are found in the electron cloud, which is the region outside the nucleus.
- The electron configuration determines the distribution of electrons in an atom's orbitals.
- Energy levels or shells in an atom are designated by the principal quantum number (n).
- Electrons in higher energy levels are farther away from the nucleus and have more energy.
- The position of an electron is described by its orbital, which is characterized by three quantum numbers.
In conclusion, understanding where an electron is found in an atom is essential for understanding the properties and behavior of atoms. The electron configuration, energy levels, and orbitals all play a crucial role in determining the position and movement of electrons in an atom. By grasping these concepts, we can better appreciate the complexities of atomic structure and the importance of electrons in determining the properties of elements.
What is the electron cloud, and where is it located in an atom?
+The electron cloud is the region outside the nucleus where electrons are likely to be found. It is not a fixed location but rather a probability distribution of where electrons are likely to be found.
What determines the energy level of an electron in an atom?
+The energy level of an electron is determined by the principal quantum number (n), which is an integer that starts from 1 and increases as you move away from the nucleus.
What is the difference between the 1s and 2p orbitals in an atom?
+The 1s orbital is spherical in shape and is closest to the nucleus, while the 2p orbitals are dumbbell-shaped and are farther away from the nucleus.



